Jonathan Clinger, Ph.D.
Assistant Professor Cancer Prevention Research Institute of Texas Scholar
Chemistry and Biochemistry

Education
Postdoctoral Researcher, Laboratory of Atomic and Solid-State Physics
Cornell University, 2019-2022
Postdoctoral Fellow, Department of Biosciences
Rice University, 2018-2019
PhD. Biochemistry
Rice University, 2012-2018
B.S. Biochemistry
Lipscomb University, 2008-2012
Experience
Assistant Professor
Baylor University 2022-present
Research
In my lab we study the structure and dynamics of proteins so we can better understand how proteins perform the physical and chemical jobs they do in the living cell. Of particular interest to us are enzymes, which are proteins that catalyze chemical reactions. Enzymes are quite amazing catalysts that biology has developed to carry out often challenging organic chemistry while being limited to primarily carbon, nitrogen, oxygen, and hydrogen, as well as a handful of cofactors. Enzymes accomplish this task due to their three-dimensional structure.
However, knowing a structure of an enzyme is often not enough to fully appreciate the catalytic mechanism (that is, how the enzyme accomplishes the chemistry) of these proteins, as their structure changes when interacting with the environment, binding substrates, and catalyzing the reaction. Therefore, my lab specializes in time-resolved methods where we capture intermediate structural states along a reaction path while it is in progress. That allows us to watch the chemistry in action and understand how the protein moves while it is performing reactions. These challenging experiments require millisecond time resolution and much of my career has been devoted to developing new techniques to observe these reactions more easily.
Current projects in my lab include:
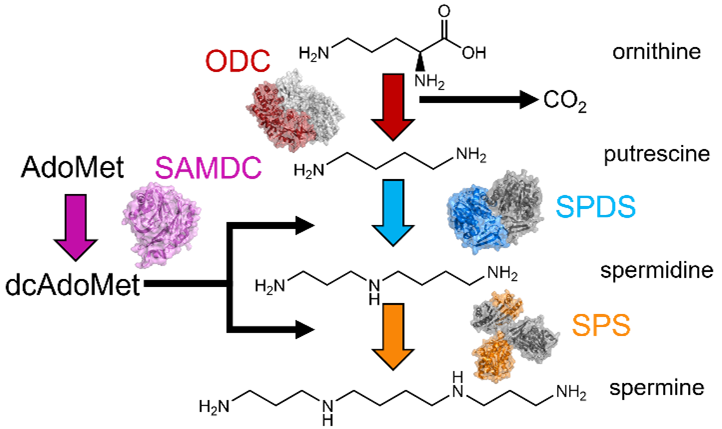
- Time-resolved and multi-temperature crystallography of polyamine synthesis enzymes ornithine decarboxylase (ODC), S-adenosyl methionine decarboxylase (SAMDC), spermidine synthase (SPDS), and spermine synthase (SPS) for rational drug design applications in cancer. The polyamine synthesis enzymes perform the series of reactions that creates the polyamines spermidine and spermine from ornithine. These enzymes are essential for normal cell growth and often become dysregulated in cancers, with heightened levels of these enzymes commonly observed in hepatocellular cancer and childhood neuroblastoma. Even though these enzymes are known to be upregulated in cancer, only ODC has an FDA approved inhibitor, with multiple substrate mimics failing in clinical trials. We anticipate with time-resolved and multi-temperature crystallography experiments that we will be able to capture previously unobserved structural states and better understand the enzymes’ behavior during their respective reactions.
With a better understanding of the structural dynamics of these enzymes, we will design new candidate inhibitors to bind more tightly to these enzymes. This project is funded by a CPRIT recruitment grant, and I am currently recruiting graduate students to work on it.
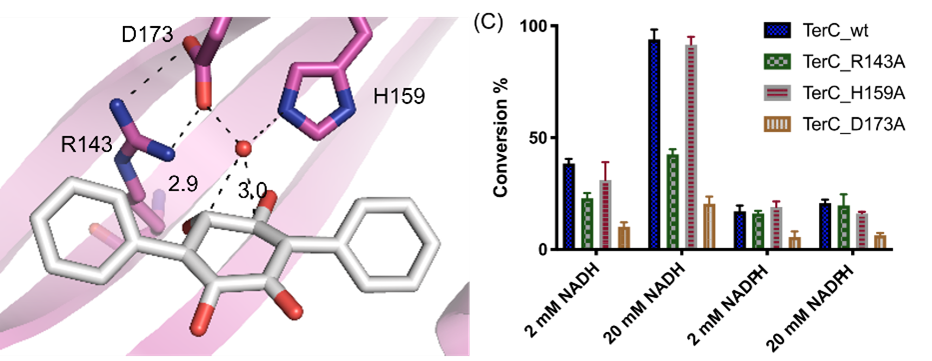
- Dynamic crystallography of natural product biosynthesis enzymes. Natural products, organic molecules synthesized by naturally occurring organisms in nature, have long been a primary source of pharmaceutical compounds. These complex compounds often require many steps to accomplish the total synthesis of in the laboratory setting. However, the microbes that make these compounds often require fewer steps and are more efficient that our processes. They accomplish this amazing feat with their synthetic enzymes. These widely varied and interesting catalysts perform reactions such as: [4+2] cycloaddition, Claisen condensation, acylation, and dehydration, to name a handful. Understanding the structure and mechanisms of these enzymes will help us be able to tweak the reactions to work on non-native substrates and produce non-native products with varying properties to enable medicinal chemistry tailoring in vivo. These tweaks can also enable synthesis of bioorthogonal metabolites that won’t interact with or disrupt the regular cellular processes while in production, enabling higher yields and fewer off target reactions.